Siemens Healthineers MedMuseum
Discover (hi)stories
- “I have lost myself, so to speak”
“I have lost myself, so to speak”
In a talk in 1906, Alois Alzheimer set out the case history of his patient Auguste Deter. She had been brought to the “Institute for the Insane and Epileptic” in Frankfurt am Main, where Alzheimer was working as a senior physician, in 1901. As part of the talk, he described the typical early signs of Alzheimer’s dementia , including memory loss, disorientation, and changes in behavior and personality. Although Alzheimer had seen these symptoms in many patients before, he was puzzled by Deter because she was just 51 years old.
Alzheimer therefore began studying the patient in detail. As well as determining her general physical condition, he conducted various cognitive tests and documented them meticulously in her medical records. These tests were based on conversations that Alzheimer held with Deter, in which he set her various exercises such as naming objects and solving simple arithmetic and writing tasks. Many of these tasks are still used today as part of standardized cognitive tests for the early detection of dementia.
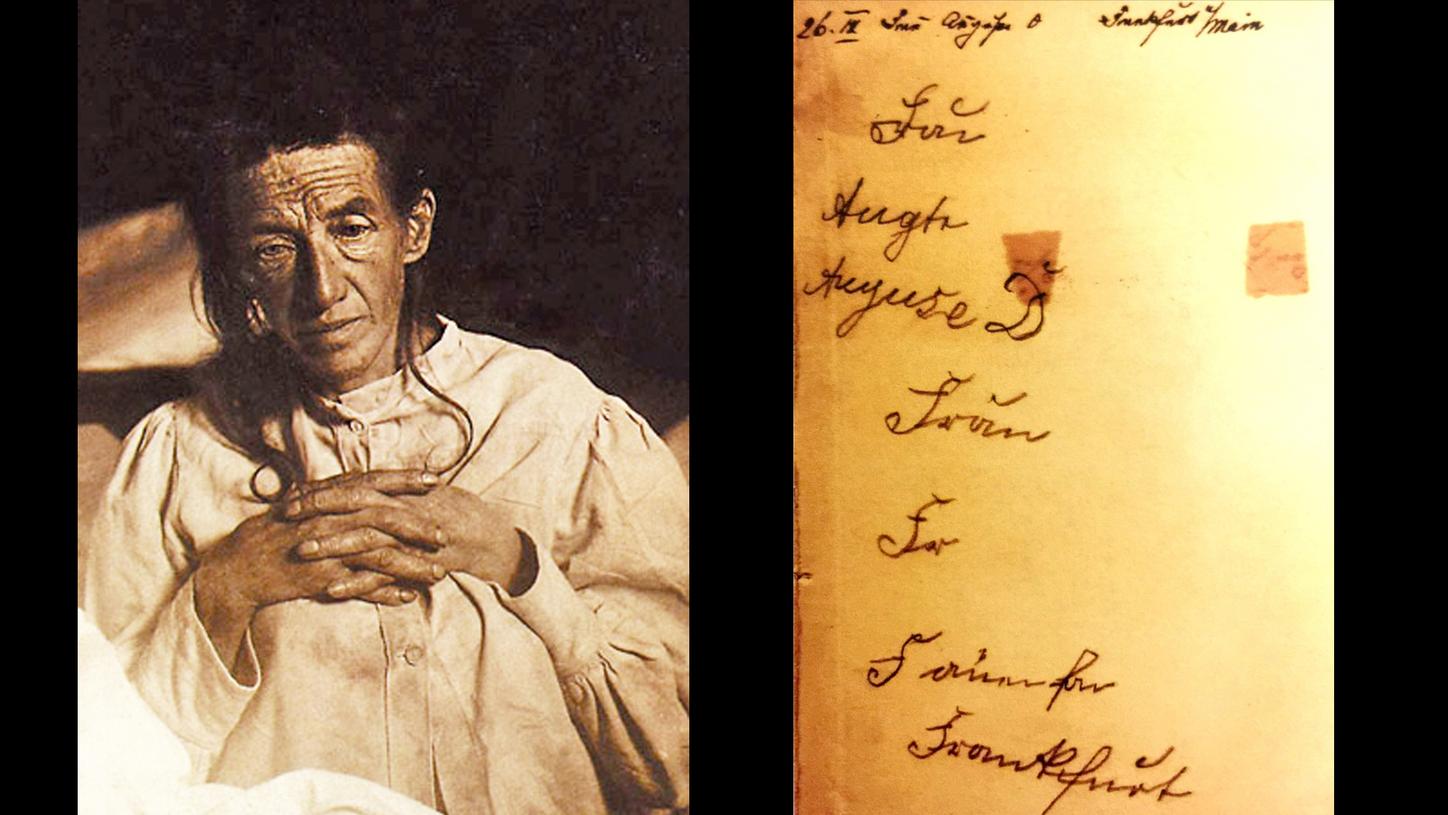
Portrait and handwriting sample from Auguste Deter
Alois Alzheimer
Alois Alzheimer (born June 14, 1864, in Marktbreit; died December 19, 1915, in Breslau, now Wrocław) studied medicine at the University of Würzburg. After completing his studies, he took up a position as a resident physician at the “Institute for the Insane and Epileptic” in Frankfurt am Main in 1888, eventually becoming a senior physician in 1896. He moved first to Heidelberg in 1902 and then, in 1903, to the Royal Psychiatric Clinic in Munich, where he studied Deter’s brain. His final stop was Breslau, where he became director of the Neurologic and Psychiatric Institute of Friedrich Wilhelm University in 1912.
What is dementia?
Dementia is an umbrella term for various diseases that affect the brain, resulting in memory and thought disorders. The most common form of dementia is Alzheimer’s, which affects between 60 and 80 percent of all people with dementia.
When Alzheimer asked his patient to write “Auguste D,” she wrote “Mrs.” but had already forgotten the rest. Alzheimer repeated her name over and over again. Instead of “Auguste,” she wrote “Auguse.” She was simply no longer able to write her full name. In a moment of lucidity, Auguste recognized her predicament: “I have lost myself, so to speak.”
Alzheimer suspected he was on the trail of a new disease. In fact, the condition had been a feature of human existence since time immemorial. Even in antiquity, a satire by the Roman poet Juvenal described dementia as one of the great terrors of old age:
But worse than any loss of limb is the failing mind which forgets the names of slaves, and cannot recognise the face of the old friend who dined with him last night, nor those of the children whom he has begotten and brought up.
Decimus Junius Juvenalis, c. 1st and 2nd century AD; translation by George Gilbert Ramsay (1918)
Searching for the causes
For a long time, the symptoms of the disease were dismissed as senile dementia. Indeed, Alzheimer himself only made a provisional diagnosis of “presenile dementia” in the case of Auguste Deter. Not content with this diagnosis, however, he continued his research. When examining Deter’s brain in the lab after her death, Alzheimer made an interesting discovery under the microscope: “Scattered throughout the cortex and particularly numerous in the upper layers, there are miliary foci due to the deposition of a peculiar substance in the cerebral cortex.” In 1984, this “peculiar substance” was identified by George Glenner and Caine Wong as an accumulation of a protein fragment known as “amyloid beta.” The question, however, was where these protein fragments came from in the first place. In 1987, a team of researchers from the University of Cologne provided an answer by showing that beta amyloid was formed by cleavage of the amyloid precursor protein. In healthy individuals, these protein fragments are broken down by enzymes and transported out of the brain. This breakdown is disrupted in Alzheimer’s patients, causing the molecules to persist and agglomerate between nerve cells — and therefore to form “amyloid beta plaques.”
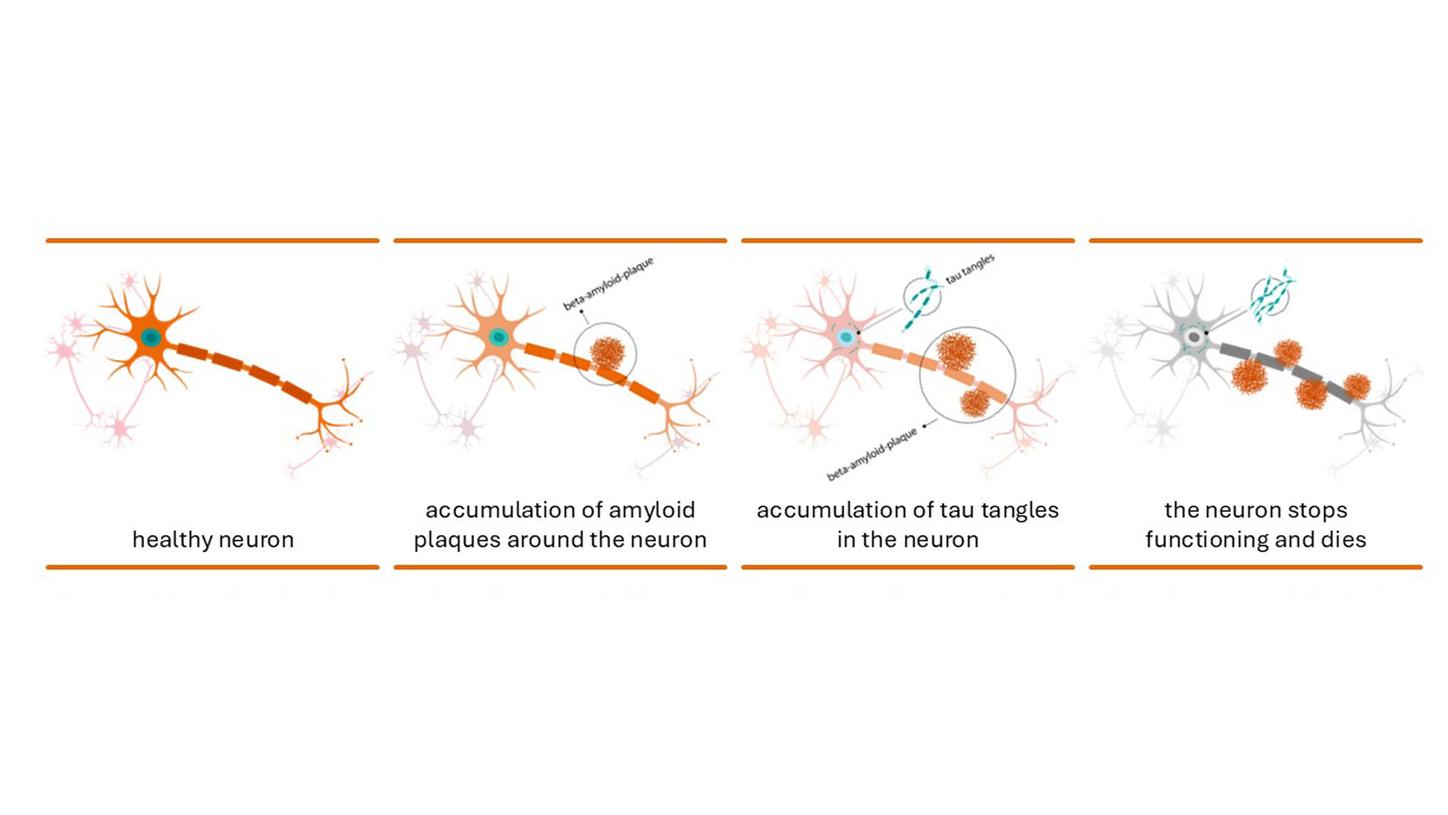
Depiction of nerve cells in a normal brain (left) and of the deposition of amyloid beta plaques and tau fibrils in the brain of an Alzheimer’s patient (middle), which lead to cell death (right),
Moreover, Alzheimer observed “very peculiar changes in the neurofibrils,” which Inge Grundke-Iqbal and her colleagues sought to clarify some 80 years later. Neurofibrils are fibers within the brain cells that help to maintain the cell structure. The research group led by Grundke-Iqbal found that one key component of neurofibrils is the tau protein. This protein stabilizes transport processes within the brain, but an abnormality in Alzheimer patients causes it to become matted into bundles — known as tau fibrils — within the nerve cells. Both tau fibrils and amyloid beta plaques play a key role in the development of Alzheimer’s disease, in that they prevent nerve cells from communicating with one another and cause them to slowly die. It is still unclear why these deposits form.
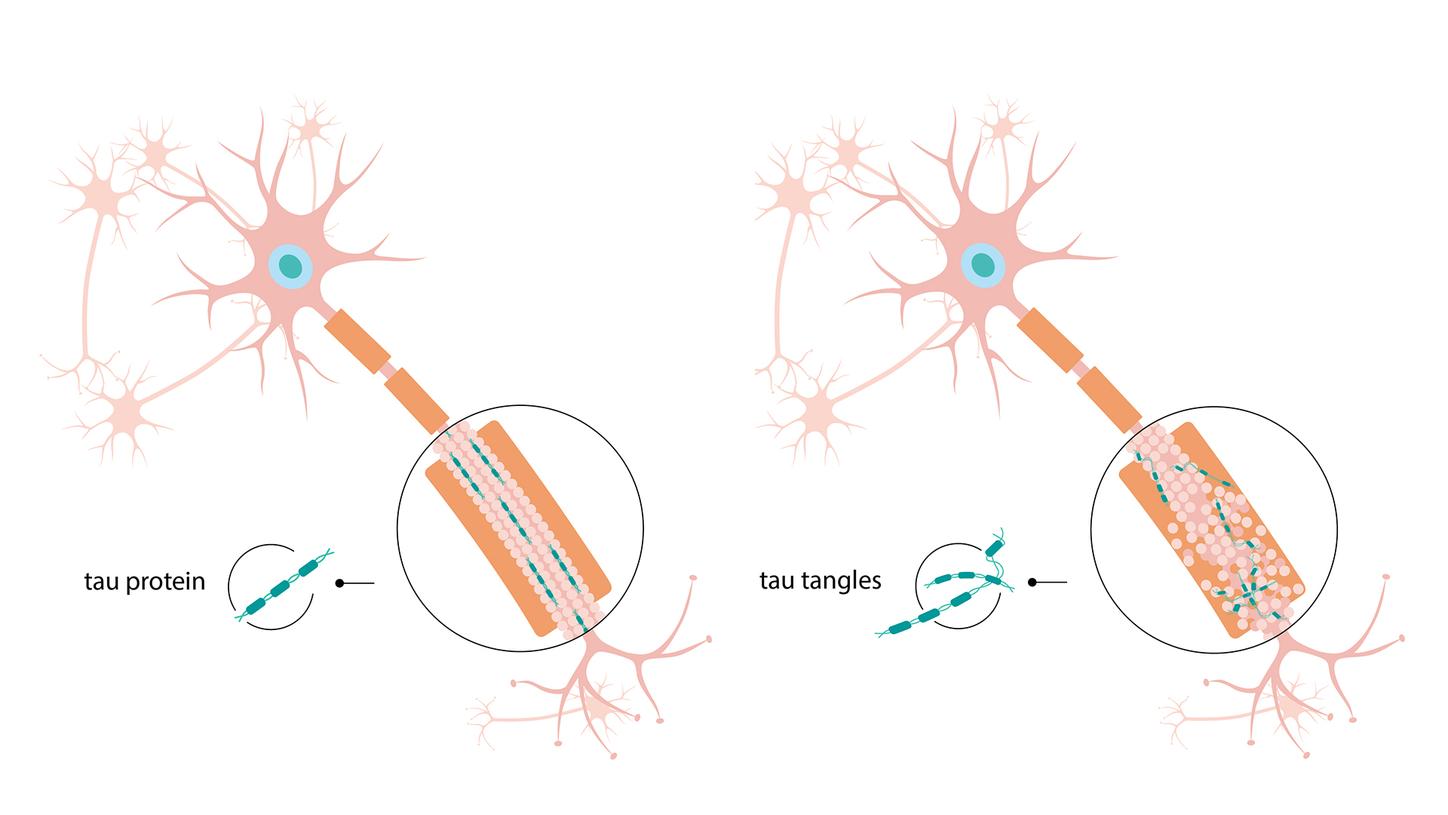
Comparison of a healthy nerve cell (left) and neuronal degeneration (right). In the latter, the tau proteins (turquoise) clump together into tau fibrils that accumulate within the nerve cells.
A Hollywood icon becomes an Alzheimer’s patient
Research into Alzheimer’s steadily gained momentum in the 1980s. Although the disease was long considered a rare condition, it has become increasingly common since the 1960s, due to longer life expectancy. The older people get, the greater the risk that they will develop Alzheimer’s. Sometimes, however, the disease can also affect younger people — as demonstrated by the cases of Auguste Deter and the American actor Rita Hayworth, who were both in their early 50s when they began showing symptoms.
In Hayworth’s case, when the disease began to manifest in the early 1970s, her symptoms included mood swings and forgetting her lines on set. Were these perhaps just the typical affectations of a Hollywood icon? As her condition worsened, she was found several times wandering disoriented through Los Angeles. It was thought she was suffering from severe alcoholism. In 1981, the correct diagnosis was finally made: Hayworth had Alzheimer’s disease. Suddenly, the media began to report on the disease, thereby raising awareness of it among the general public.
Hayworth’s case shows just how difficult it still was to diagnose Alzheimer’s in the 1980s. Ultimately, a complex method consisting of various examinations and cognitive tests only allowed physicians to rule out other diseases. There was no way to diagnose Alzheimer’s at an early stage either in the blood or in a computed tomography (CT) or magnetic resonance imaging (MRI) scan. Indeed, the disease could only be observed in clinical images once it was too late and had already caused shrinkage of the brain. It wasn’t until the development of positron emission tomography (PET) and special tracers that physicians had a way of making a clear diagnosis. In the mid-1990s, this method was complemented by another diagnostic technique, known as the cerebrospinal fluid test , after researchers found that Alzheimer’s could be detected by analyzing this fluid. Both of these techniques have now become standard procedures for the early detection of Alzheimer’s and can be carried out using systems from Siemens Healthineers.
What is PET, and what are tracers?
Positron emission tomography (PET) is a nuclear medical technique that visualizes metabolic activity in the body. It involves injecting the patient with a substance known as a tracer. This is a weakly radioactively labeled substance, such as glucose, that takes part in metabolism. The PET scanner then visualizes the tracer’s distribution in the body.
What is a cerebrospinal fluid test?
This examination involves inserting a cannula into the patient’s spinal canal in order to extract cerebrospinal fluid. The fluid is then analyzed in the lab using systems such as Atellica® Solution. If the patient is suffering from Alzheimer’s, the fluid will contain altered concentrations of the amyloid beta and tau proteins that typify the disease.
Development of positron emission tomography
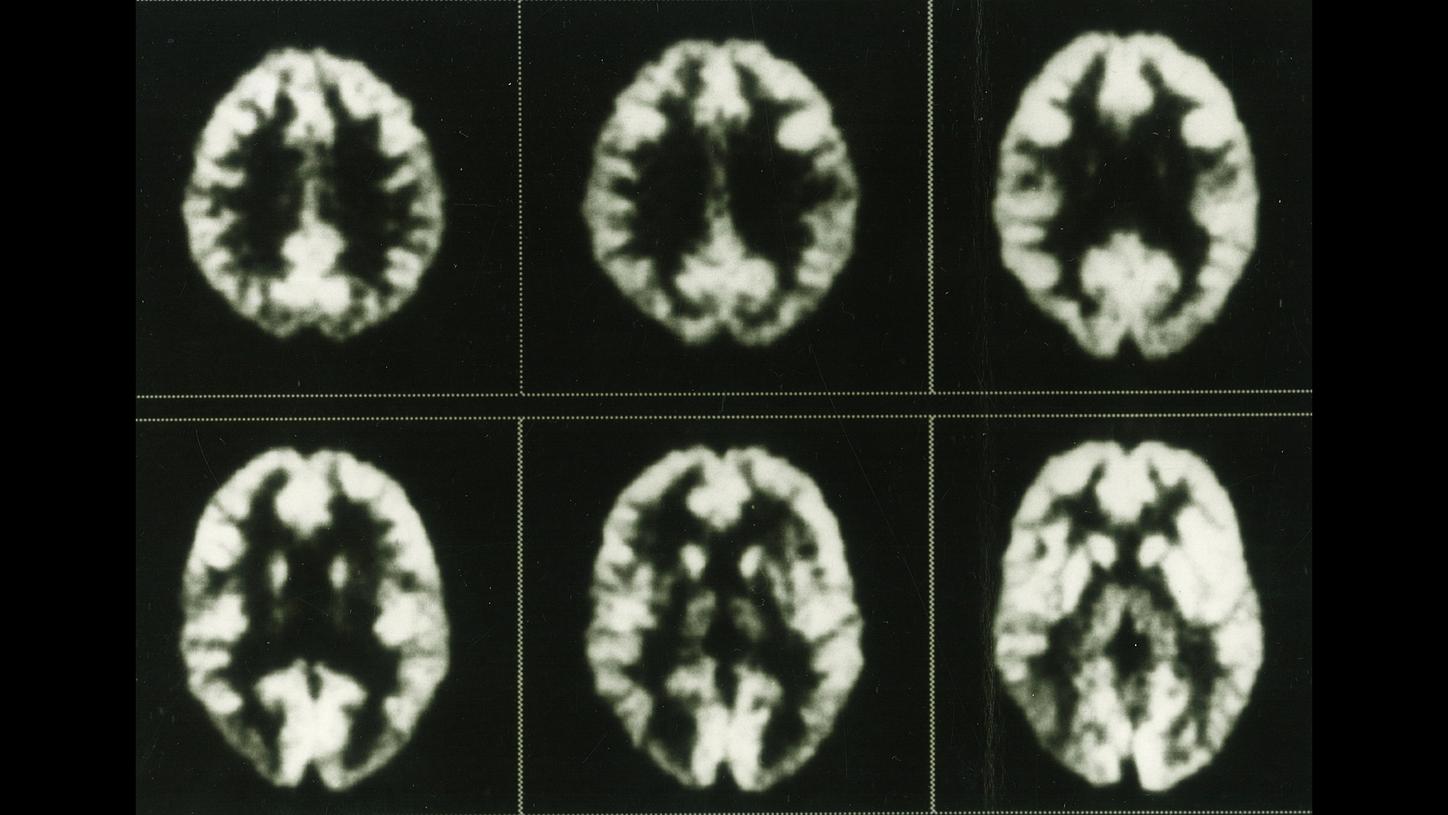
When PET scanners were first developed at the start of the 1970s, even early images from prototypes allowed physicians to observe glucose metabolism in the brain. This series of images, recorded using the ECAT III in the mid-1980s, shows the distribution of fluorodeoxyglucose in a healthy brain.
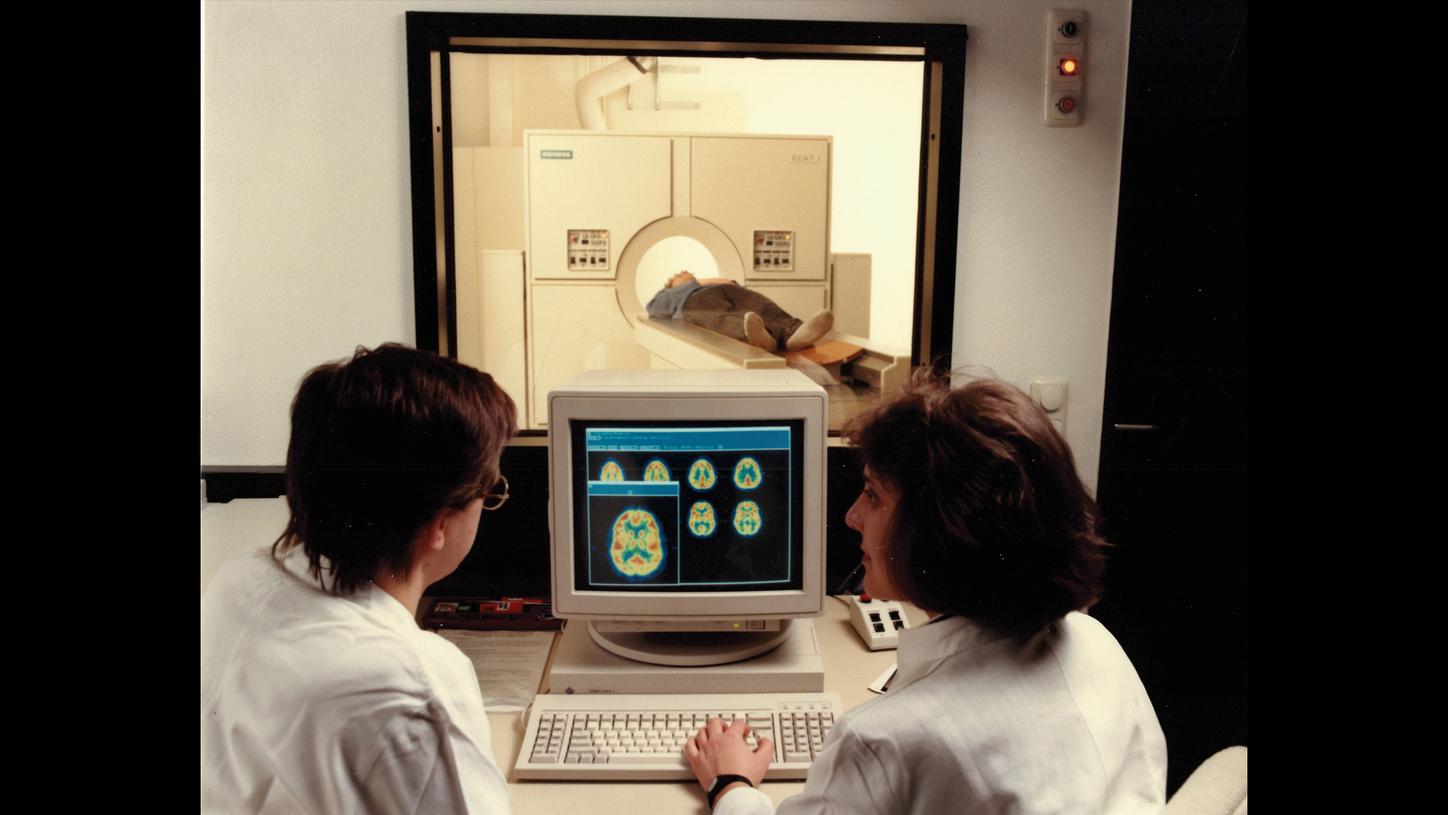
PET was also used to diagnose Alzheimer’s from the 1980s onward, but it wasn’t a routine procedure yet and was primarily used for research purposes.

In the late 1980s, PET was still principally a tool for research and was rarely used in hospitals because there were only a small number of cyclotrons that could produce the tracers. The development of small, affordable cyclotrons allowed tracers to be produced directly in hospitals. In parallel, an extensive distribution network was developed in the form of PETNET so that tracers could be delivered to practices quickly.
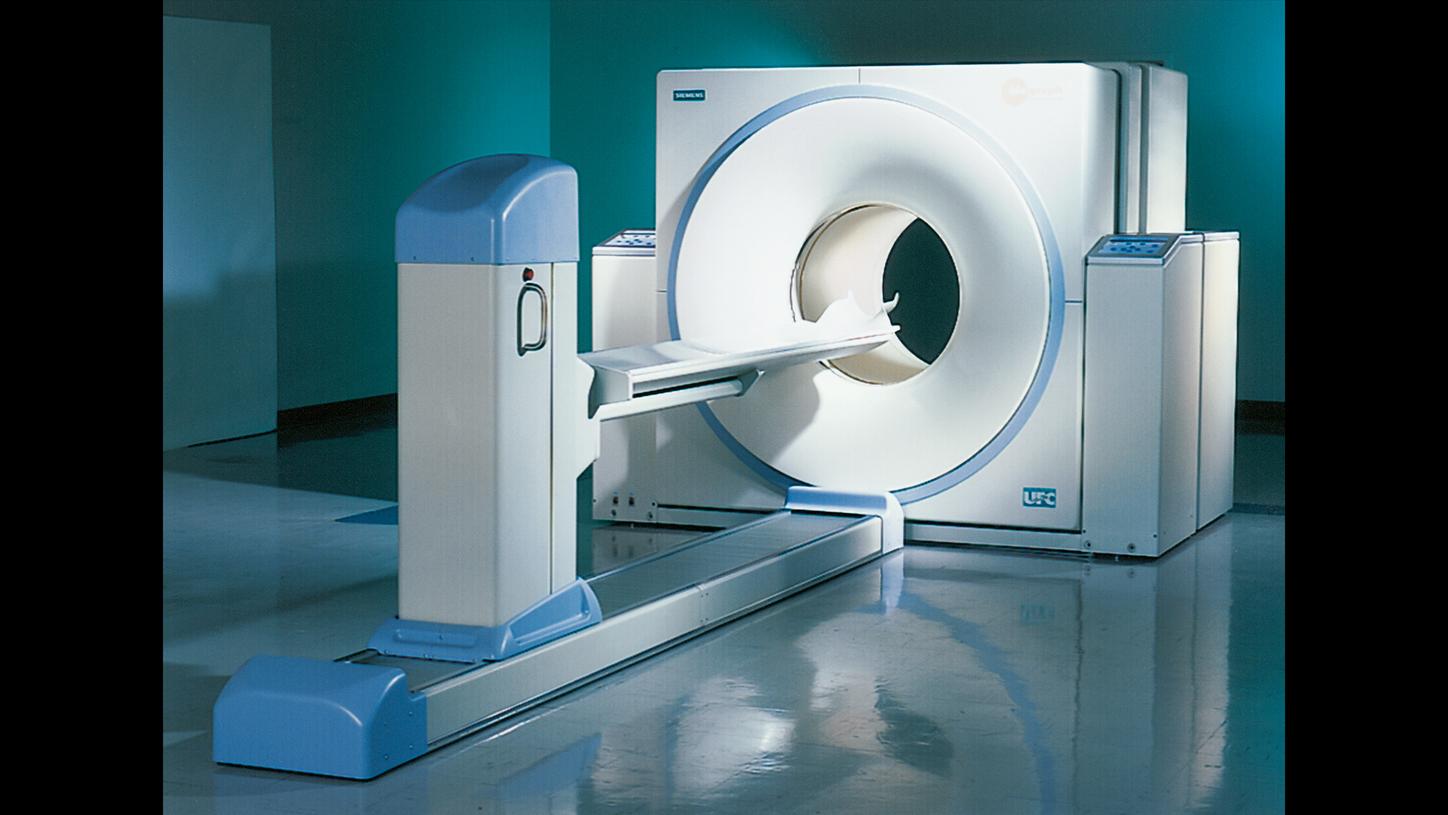
PET shows metabolic activity but does not visualize the patient’s anatomy. Since the year 2000, it has been possible to do both things at the same time with Biograph, which combines PET and CT imaging within a single device.
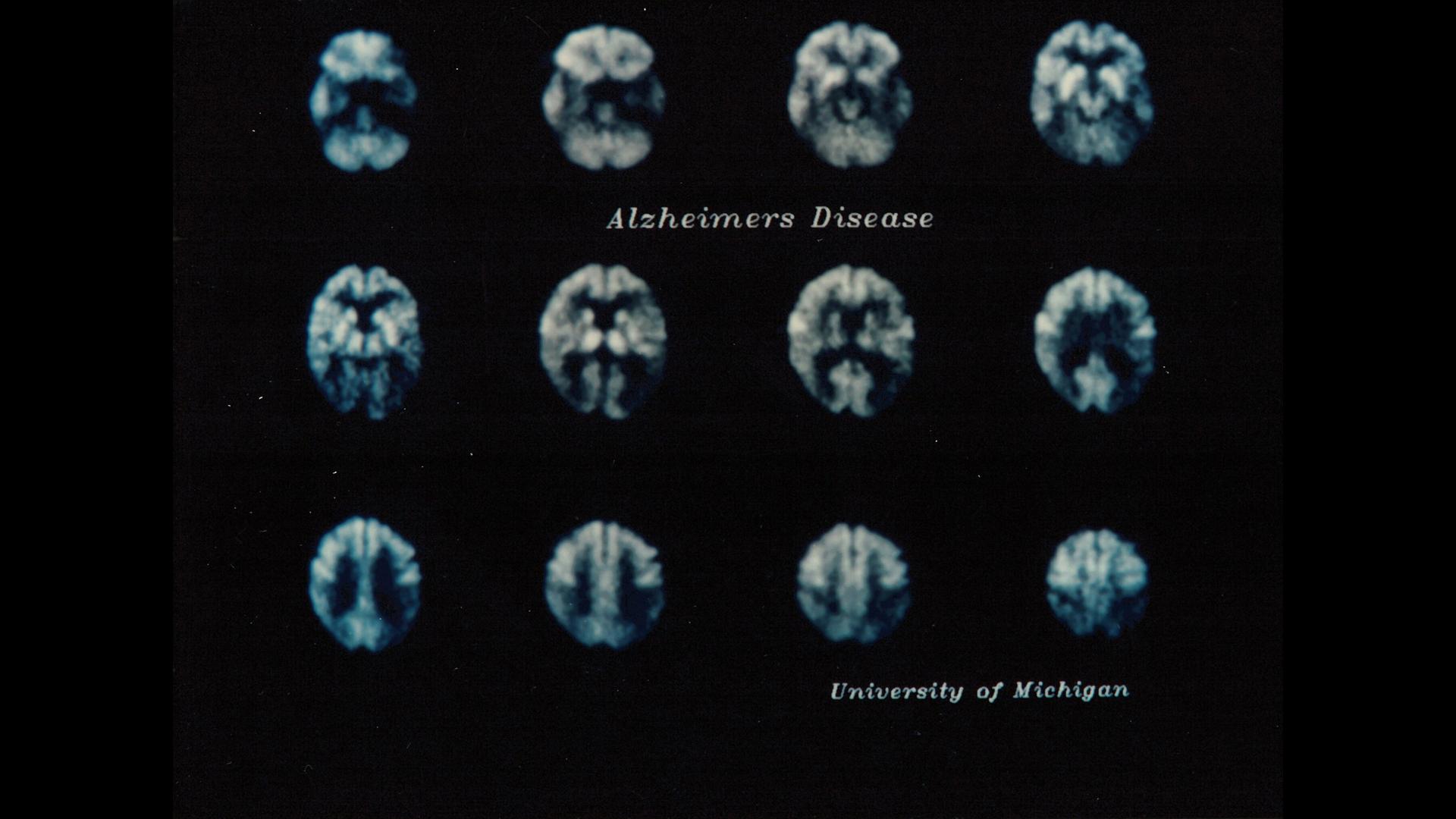
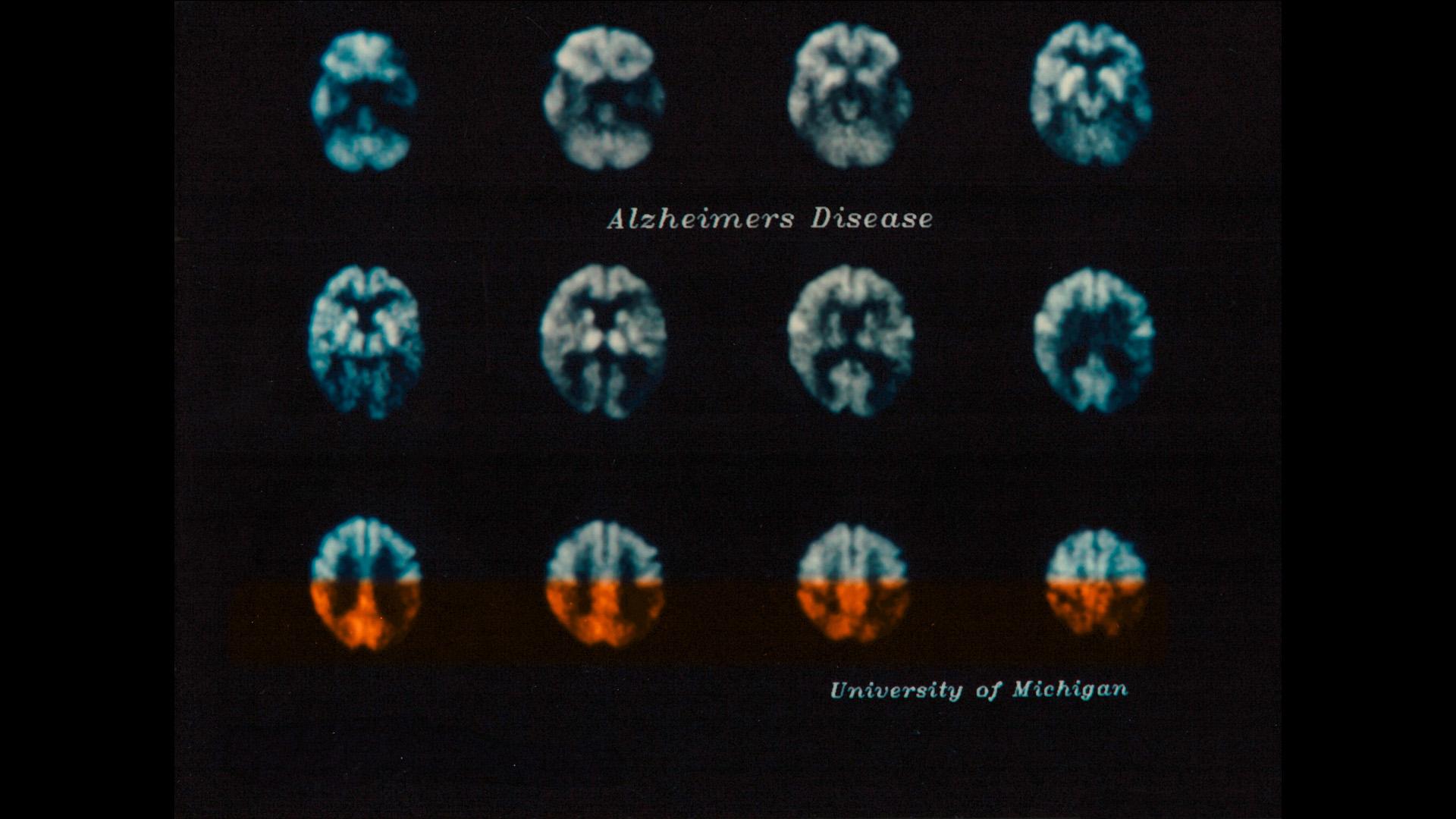
These PET images from 1989 show reduced glucose consumption due to disruption in nerve cell function in certain regions of the brain (marked in orange), which can be an indicator of Alzheimer’s disease.
PET imaging of glucose metabolism in the brain visualized the effects but not the causes of Alzheimer’s. That changed in the early 2000s, when a team of researchers at the University of Pittsburgh began developing a tracer that revealed amyloid beta plaques in the brain. In parallel, a team of scientists at Siemens were researching a biomarker that could be used to identify tau deposits. Using these tracers and modern PET/CT scanners, Alzheimer’s could now be detected early — before the damage to the brain had reached an advanced stage. The question remained, however: What use is there in detecting the disease early if there is no prospect of curing it?
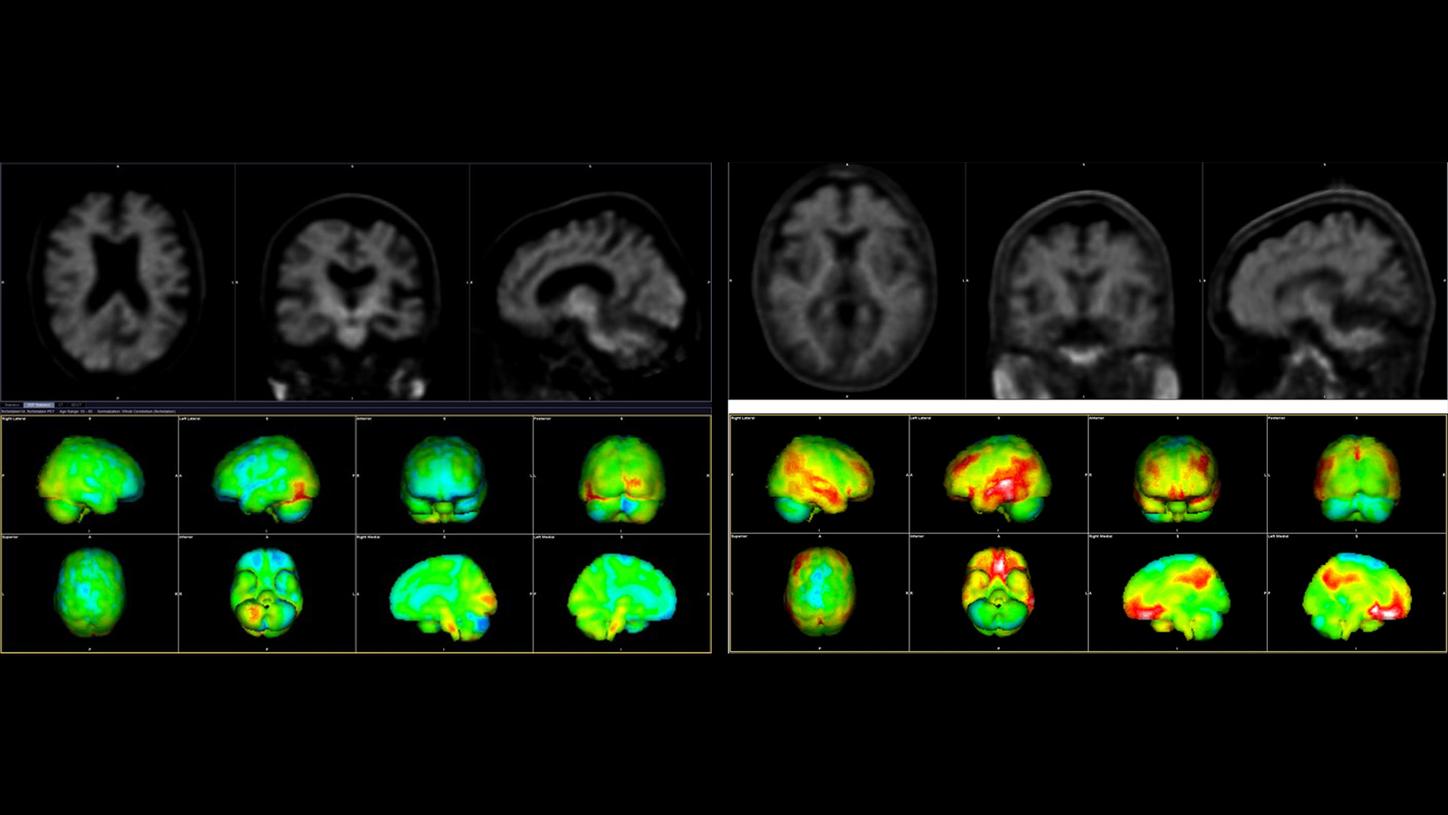
Amyloid PET/CT images to detect amyloid beta plaques (marked in red). The image on the left shows the brain of a healthy older person, while the image on the right shows that of an Alzheimer’s patient.
Data courtesy of Wentworth-Douglas Hospital, Dover, New Hampshire, USA
I now begin the journey that will lead me into the sunset of my life.
Ronald Reagan, 1994
It was with these moving words that, in 1994, former U.S. President Ronald Reagan concluded his letter to the public, announcing that he was suffering from Alzheimer’s. To this day, anyone receiving an Alzheimer’s diagnosis faces the same journey, in which they will lose themselves completely. There is still no cure for the disease, but there is some cause for hope: Since 2023, two antibody-based drugs have been approved in the US that target and break down amyloid beta plaques in the brain.
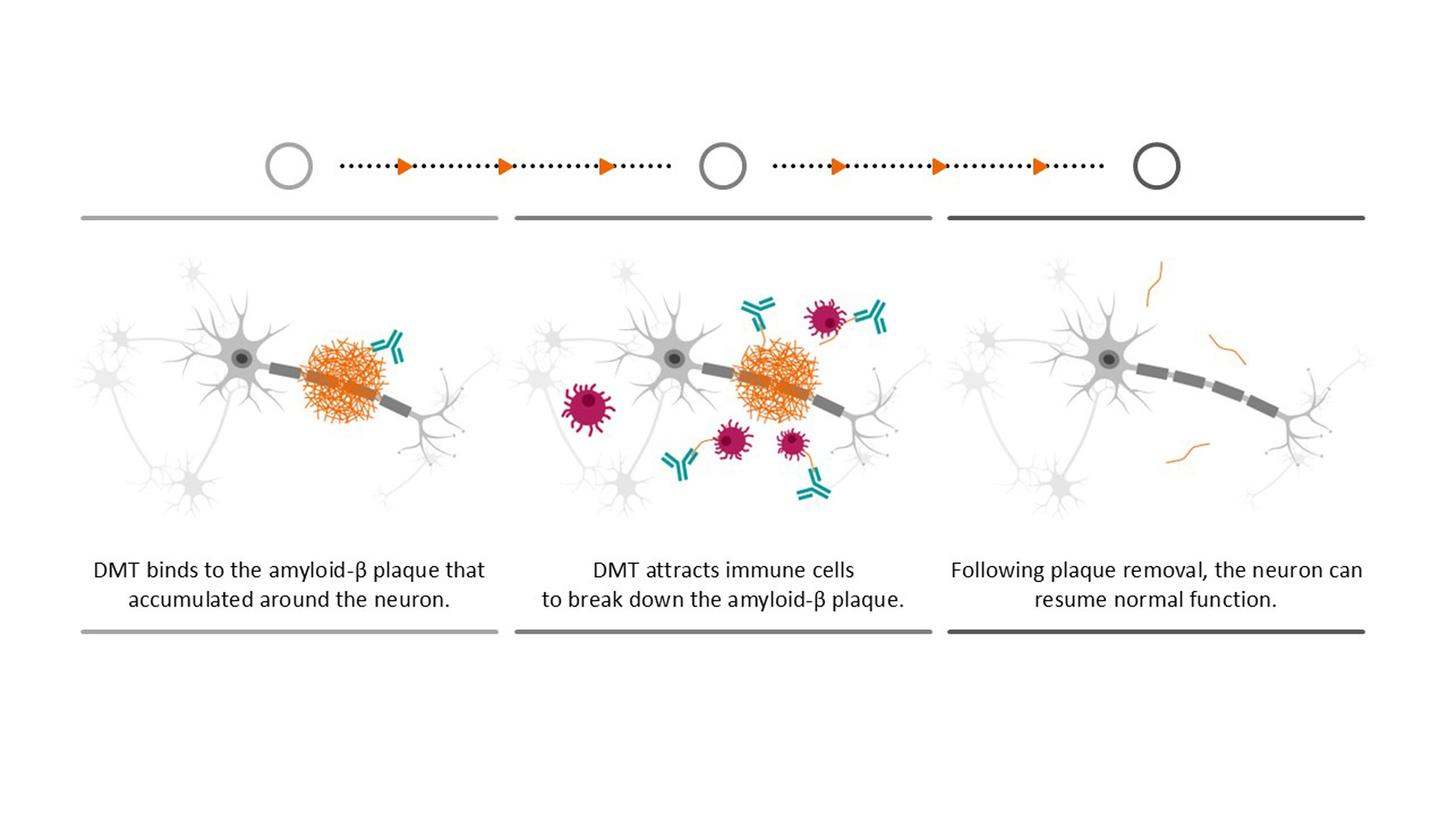
Rather than curing the disease, the drugs can only slow down its progression — and they are only suitable for a small group of patients with very early-stage Alzheimer’s. Moreover, the numerous side effects mean that patients must be monitored closely over the course of treatment and require regular MRI scans. Nevertheless, the drugs offer a glimmer of hope and are just one of many options currently being explored with a view to not only slowing down the disease but also, one day, finding a cure. Of course, Alzheimer’s research currently centers around understanding the disease itself, because there are many unanswered questions. With our technologies, we support researchers and accompany patients on their journey. We want to give people more time with their loved ones and help them live independently and according to their own wishes for as long as possible.
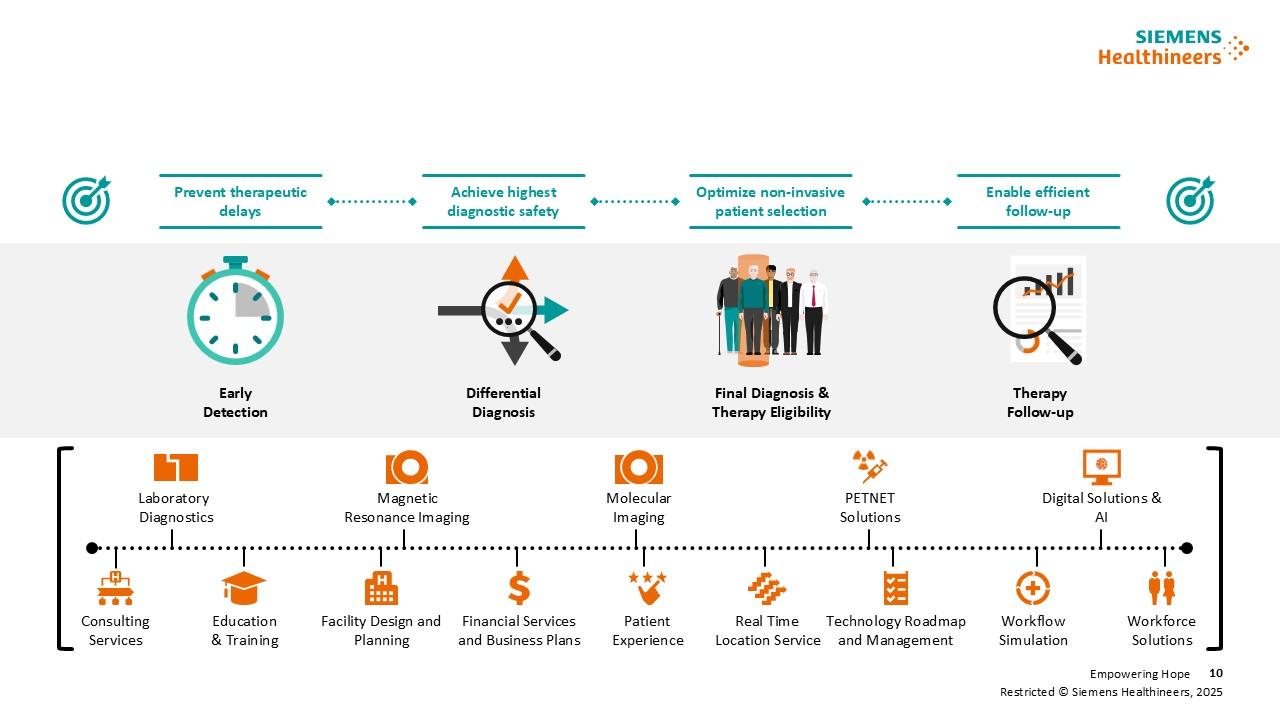
Clinical solutions from Siemens Healthineers support the entire Alzheimer’s care pathway: from early detection to differential diagnostics and treatment monitoring.
Expert for History Communication and Historian at the Siemens Healthineers Historical Institute






